GROUNDWATER TREATMENT TECHNOLOGY
Iron and mangan treatment technology, pH elevation without chemical usage, will help water supply companies save a significant amount of raw material and fuel costs, as well as reduce water consumption during the treatment and operation processes at centralized groundwater treatment plants.
Due to its characteristic of being deep within the ground, groundwater lacks oxygen and may contain substances such as iron (Fe), manganese (Mn), ammonia (NH4+), methane gas (CH4), and hydrogen sulfide (H2S). Understanding this issue, the treatment technology proposed by the company includes an initial step of aeration and gas exchange. The goal is to provide oxygen and remove carbon dioxide (CO2) gas present in the incoming water. At the interface between water and air, a continuous gas stream moves from the air into the water and vice versa. When the contact surface area between water and air is sufficiently large and the gas exchange time is long enough, the amount of gas in the water reaches equilibrium with the amount of gas in the air. Supplying oxygen into the water has the effect of oxidizing dissolved substances such as iron (Fe2+), manganese (Mn2+) into insoluble forms, and ammonia (NH4+) into nitrate (with the help of Nitrosomas and Nitrobacter bacteria). Additionally, through aeration, harmful gases such as CO2, methane gas (CH4), and hydrogen sulfide (H2S) are partially removed.
The gas exchange process is slow, but it can be accelerated by having a wide and open contact surface, prolonged contact time, and renewing the water-air interface more frequently. If aeration is conducted under the specified conditions, the following reactions will occur:
SO4 + 5H2 → H2S (↑ gas) + 4 H2O
H2CO3 → CO2 + H2O
CH4 + 2O2 → CO2 + 2H2Os
4Fe2+ + O2 + 18H2O → 4Fe(OH)3↓ + 8H3O+
NH4+ + 2O2 + H2O → NO3– + 2H3O+
2Mn2+ + O2 + 6H2O → 2MnO2↓ + 4H3O+
The reactions mentioned above rely entirely on the principles of biology, mechanics, absorption, and activation. The working principle is based on simultaneous and interdependent mechanisms. There is no intervention of chemicals (oxidizing agents such as chlorine, KMnO4, etc.). This technology utilizes natural quartz sand as the filter medium and applies forced aeration and an oxygen tower. The treatment process follows the following diagram:
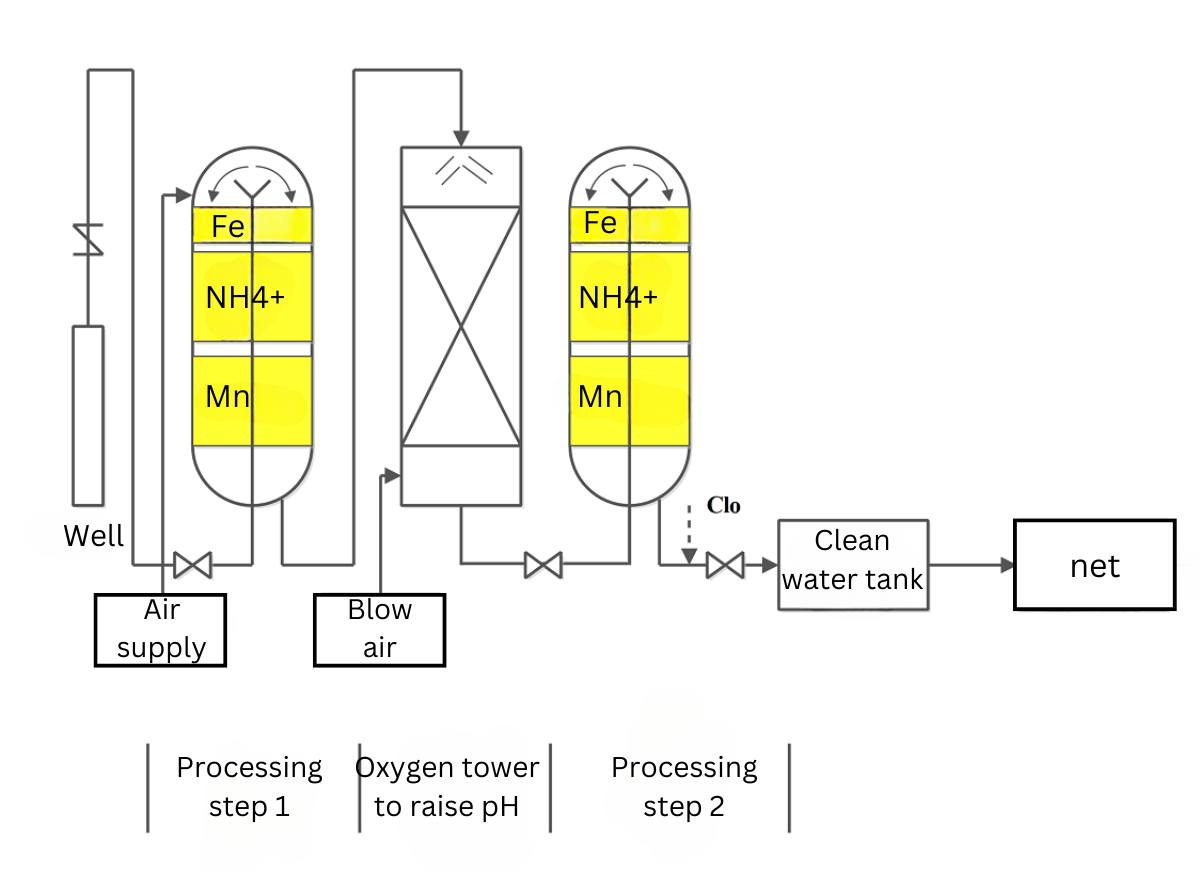
The treatment technology of the system will go through 2 processes:
Treatment Step 1: Primarily involves chemical reactions, releasing gases such as H2S, CO2, and converting the majority of Fe2+ to Fe3+ (achieving up to 99% iron removal depending on the iron content in the raw water), partially reducing NH4+ and Mn2+, all of which are retained entirely in the sand filter layer.
Subsequently, the water flows through an aeration tower: The pH is raised as a large amount of CO2 gas is released due to the increased contact surface area between water and oxygen. In the oxygen tower, the contact surfaces are continuously renewed, and the contact time is extended.
Treatment Step 2: The remaining Fe2+ (if any) is completely converted to Fe3+, and Mn2+ is transformed into Manganese dioxide, which is retained entirely in the sand filter layer that they pass through.
+No abnormalities were detected, and the pH value of 7.53 falls within the safe range (6.5-8.5).
Through practical projects implementing the 2-step treatment model, the treatment system has affirmed its outstanding advantages. The aforementioned treatment system not only eliminates the need for chemicals but also effectively removes iron and manganese while adjusting the pH as required. The water quality after treatment remains stable and meets the requirements of QC 01/2009 by the Ministry of Health. The operation is not overly complex and does not require high-level expertise, resulting in low operational management costs and savings in natural resource material costs. Additionally, it requires minimal maintenance and has low investment costs.

Iron and Manganese filtration system and chemical-free pH adjustment with a capacity of 6,000 m3/day







